Production of API-loaded PLGA Nanoparticles for Drug Delivery Utilizing Microfluidic Technology
Background
Blacksheep’s Science team successfully undertook a customized project in collaboration with an academic customer. The customer is interested in using a biocompatible poly (lactic-co-glycolic acid) (PLGA) polymer to encapsulate their hydrophobic anticancer Active Pharmaceutical Ingredient (API) for drug delivery, hence improve its solubility, stability, and bioavailability. By employing cutting-edge microfluidic technology, we successfully generated API-loaded PLGA nanoparticles (NPs) with an average size of around 80 nm while achieving an encapsulation efficiency of over 90%.
The results showcased substantial optimization of nanoparticle size, morphology, encapsulation efficiency and drug release profile, demonstrating the potential of microfluidic technique for scalable and high-quality fabrication of API-loaded PLGA nanoparticles.
The journey of solution
Results
Microfluidic generation of API-loaded PLGA nanoparticles
Nanoparticle-based polymers have become a key focus in drug delivery and medicine development due to their ability to target specific areas of the body and protect drugs from degradation. The conventional batch synthesis of polymeric nanoparticles, such as PLGA NPs, has limitations in terms of quantity, control over particle attributes, consistency, optimization, and scaling up production. As an alternative, this project utilizes microfluidic technology driven by pressure pumps to produce API-loaded PLGA NPs, providing better control over the synthesis process and maintaining consistent conditions.
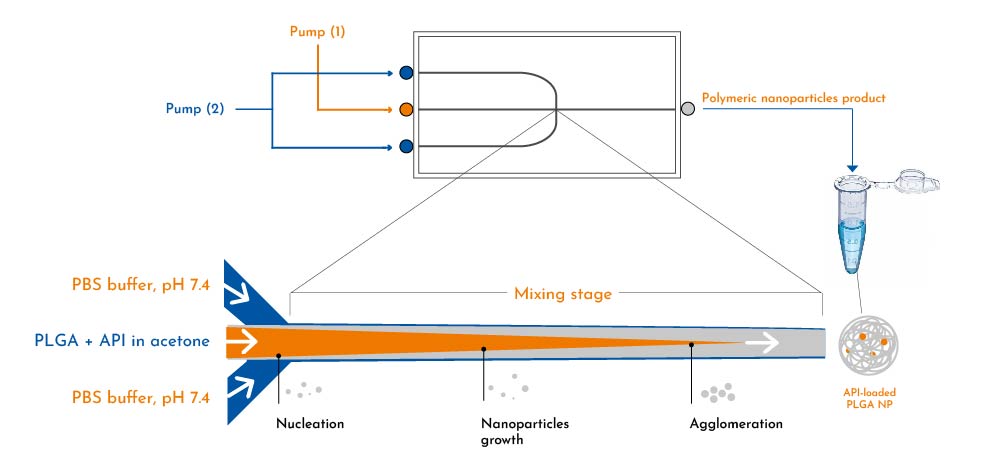
These PLGA nanoparticles carrying drug are formed by solvent diffusion through a glass microfluidic chip, where the mix of PLGA and hydrophobic API in an acetone solution and the antisolvent (PBS buffer) containing the stabilizer polyvinyl alcohol (PVA) undergo effective mixing at the junction-chip, yielding highly monodisperse nanoparticles with uniform growth histories (Scheme 1). This approach simplifies the optimization of critical quality attributes for these particles.
Optimization of API-loaded PLGA NPs for payload and release profile
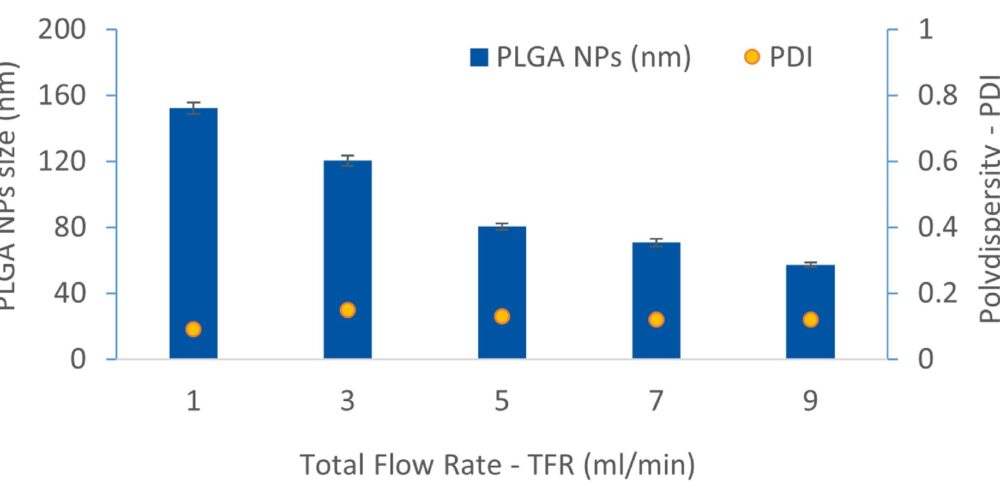
It is well-known that the assembly process of nanoparticles is complex and difficult to regulate, requiring significant resources and effort for optimization. By utilizing a 100-µm microfluidic chip, we conducted experiments with various flow rate ratios (FRR) and total flow rates (TFR) to investigate their impact on the size and dispersion of polymeric NPs. Our finding demonstrated that increasing the TFR from 1 to 9 ml/min while maintaining a constant FRR of 3:1 consistently reduced the NPs size from 153 nm to 68 nm and resulted in an average PDI of approximately 0.13 (Figure 1). By precisely controlling variables such as drug and polymer concentration, microfluidic chip junction size, FRR and TFR, we successfully achieved API-loaded PLGA NPs with the size of approximately 80 nm and an average PDI below 0.1 to meet the customer demand.
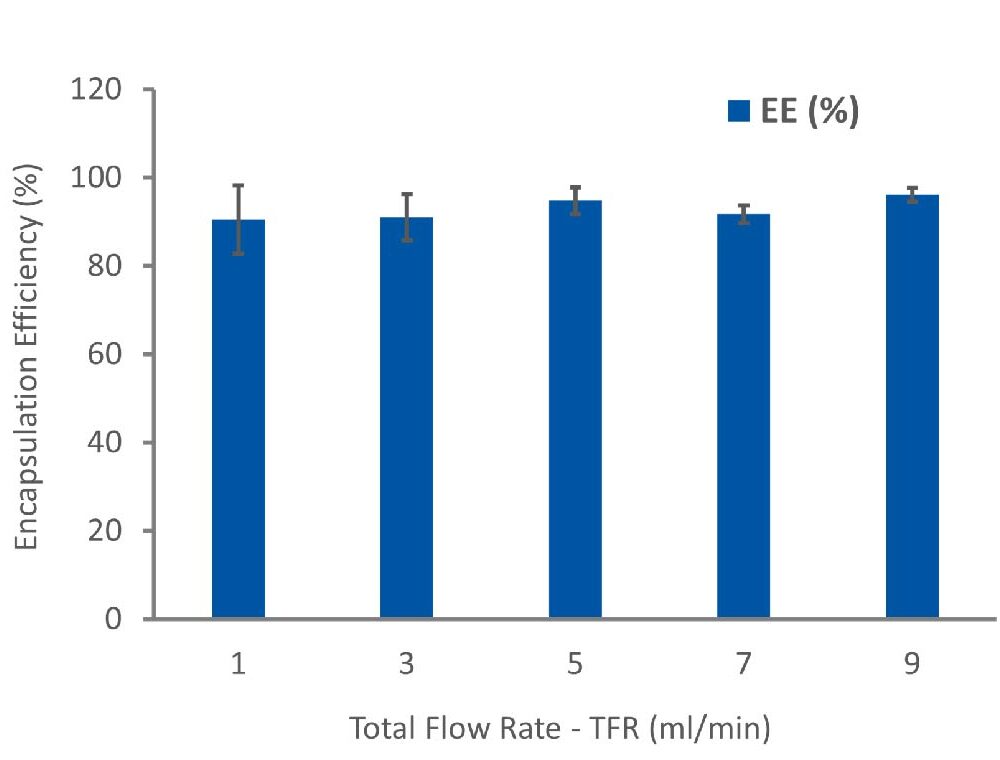
The drug loading efficiency (EE%) within PLGA NPs, was investigated using an indirect method showing that over 90% of the hydrophobic drug was successfully encapsulated within the NPs, minimizing the loss of the valuable API during the encapsulation process (Figure 2). Furthermore, we conducted a release study to assess the release profile of the loaded drug over a 96-hour period. This study further confirmed the controlled and sustained release potential of the developed API-loaded PLGA nanoparticles, ultimately reducing the required dosage and frequency of drug administration processes.
Production of API-loaded PLGA NPs
To meet the customer’s requirement for producing a minimum volume of API-loaded PLGA NPs, equivalent to 1000 doses for each potential nanoparticle product intended for in vivo studies, we utilized optimized conditions. By applying the fixed FRR at 3:1, TFR at 5 ml/min, and maintaining PLGA and drug concentrations at 2% and 0.5% (w/v), respectively, we effectively implemented a scaled-up production process for drug-loaded PLGA NPs. The pump system’s capacity was able to increase to 0.6 L/h, enabling the continuous manufacturing of nanoparticles carrying the drug in multi-liter quantities for pre-clinical testing. This approach ensures both cost-efficiency and the maintenance of high-quality NPs.
Challenges
The journey was far from straightforward, as it presented a unique set of remarkable challenges.
Unique characteristics of the API
The client’s hydrophobic drug faced solubility challenges, complicating its transport into the body. Loading the drug within polymeric NPs was hindered by unknown properties and interactions with the polymer matrix. Selecting an ideal delivery system proved challenging; prior attempts with chitosan, hydrogel, and PEGylation were unsuccessful. Our proposal involved loading the drug into a PLGA matrix to create API-loaded PLGA NPs. We screened multiple PLGA polymers for the optimal candidate, utilizing our expertise in microfluidic technology for efficient testing.
Communicating the unpredictable nature of scientific research
As part of the research, we had many carefully planned, controlled, and optimized experiments that did not lead to positive outcomes. To effectively communicate these results to customers, we developed a documentation system and tracked our experiments meticulously. We also had biweekly meetings with customers to discuss the results promptly. When needed, we adjusted our experiment design, taking into account the new results, ensuring that we have the best chances to deliver the project, while adhering to the timeline and preserving the project’s integrity.
Delay of chemicals shipping
Once we embark on the project, availability of chemicals, especially novel APIs from customer is extremely important. However, cross-border conditioned shipping is very time-consuming and requires lots of attention to regulations and declaration. We had to forecast the use of chemicals from the beginning of the project and always maintained our stock at a high level. We also partnered with the most specialized, efficient forwarders to enhance transportation. This incurred additional costs for the project but help us keep the timeline and achieve the best results.
Get started today
To find out how to partner with Blacksheep, send us your information.
